Metals and Minerals in Medical Implants
Numerous different metals, either alone or as part of an alloy, are included in the design of medical implants and non-essential metals has toxicity and bioaccumulation effects.
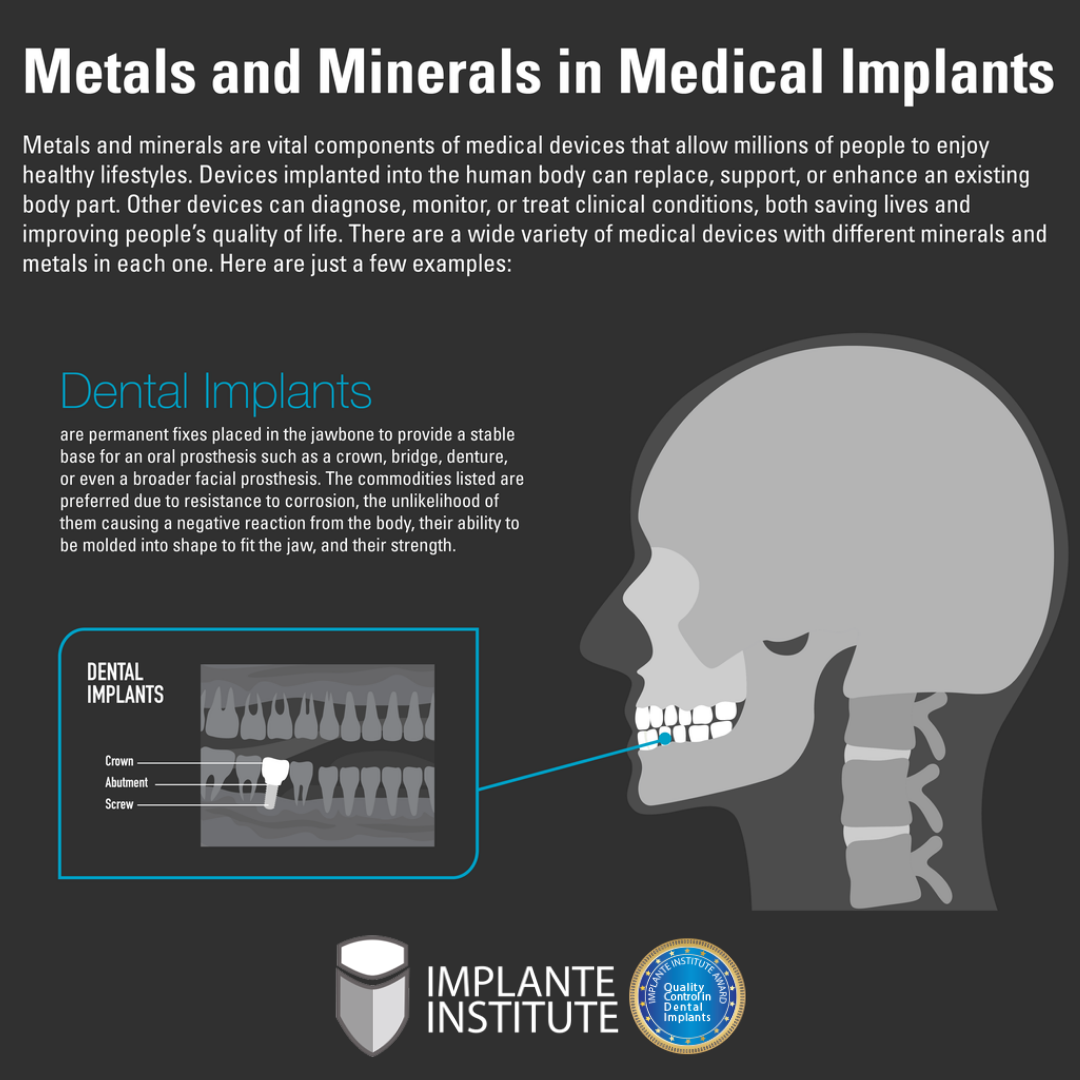
For more than 100 years, metals and metal alloys (a combination of metal elements) have been commonly used for a host of medical implant applications across most medical specialties.
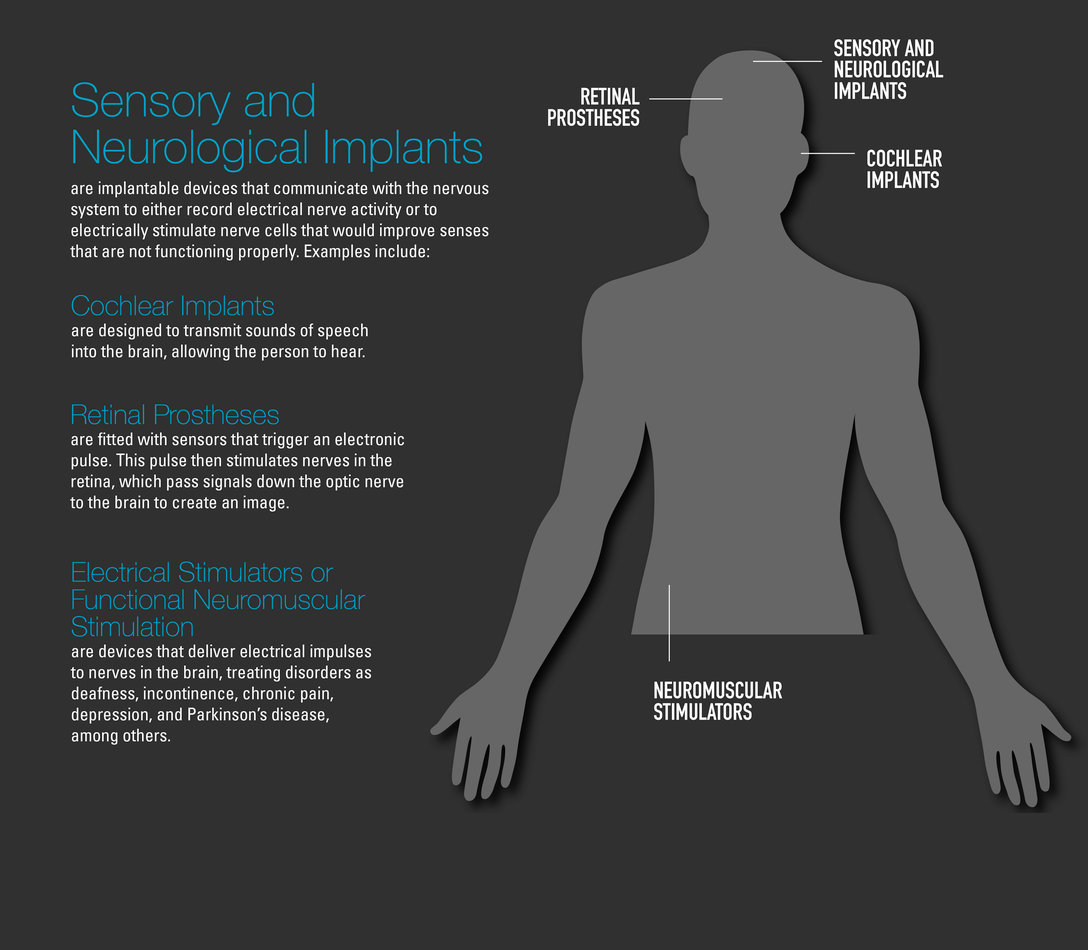
The clear majority are regulated by the Food and Drug Administration (FDA) as Class II (moderate risk) devices and cleared for marketing through the premarket notification [“510(k)”] pathway after demonstration of “substantial equivalence” to a legally-marketed Class II device or granted a De Novo request, or as Class III (higher risk) devices approved through the Premarket Approval Application (or PMA) process after demonstration of a reasonable assurance of safety and effectiveness. As part of their premarket evaluation, these products undergo a battery of nonclinical (bench and/or animal) tests – often following specific FDA guidance documents or national/international standards.

These results, along with clinical data in certain circumstances, are provided to, and reviewed by FDA prior to market authorization. In the past several years, FDA has undertaken extensive postmarket reviews of data associated with specific metal-containing implants after safety concerns were raised including for metal-on-metal (MoM) total hip arthroplasty (THA) systems and the Essure System for permanent birth control.1 In those cases, the potential role of metal components in the development of local and systemic adverse events had been questioned.
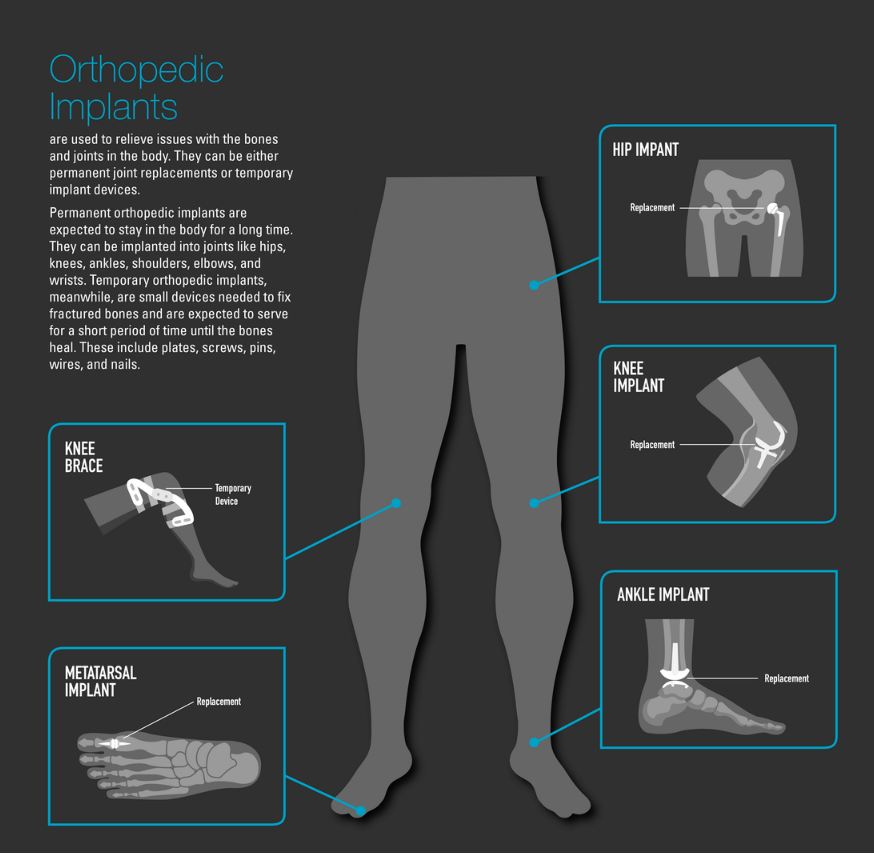
In the former example, metal wear/debris was determined to be associated with the occurrence of local pseudotumors and aseptic loosening of the prosthesis with subsequent need for revision. Some have also raised concerns over the association of elevated metal ion levels with neurological events. For the latter (Essure System), the patient-reported associations with broad systemic signs/symptoms (considered by some to represent “allergic” or “hypersensitivity” reactions) are less clear.
Together, these issues have raised questions about how an implant or insert recipient’s immune system may respond to the presence of metal in/from the device and to what degree, if any, that response may produce clinically significant signs, symptoms or adverse outcomes.

Implants are routinely used to address many different conditions in almost every medical specialty. Many of these devices allow physicians and patients significant, effective advantages over alternative surgical and medical options, and may provide a treatment when no other option exists.
These advantages have led to continued interest in the development of implanted devices over the last century. The term “implant” refers to a wide variety of different medical devices, from solid metal implants, such as orthopedic plates/screws, to bioelectronics such as pacemakers or neurostimulators.
Some implants are temporary and may be designed to be removed or replaced, while others are meant to be permanent (i.e., not intended to be removed). For this paper, implants are defined as medical devices that are placed into a surgically or naturally-formed cavity of the human body and are intended to remain there after the procedure for an extended period. This review primarily focuses on implants with metal parts or components that have contact with body tissue. Implant components based on metallic elements are chosen as a biomedical material because they have numerous advantages over other materials, including high mechanical strength, durability, good thermal and electrical conductivity, ductility, and chemical/biological compatibility.

However, the types and uses of early devices were limited due to a lack of available materials. It has only been in the last 100 years that implantable medical devices have become commonplace in healthcare. The first attempts at using metal implants outside of dental implants occurred in the late 19th century with the development of the “Lane Plate,” which was an internal fixation plate used to treat bone fractures (Lane 1895; King 1959; Uhthoff, Poitras, and Backman 2006).
The development of aseptic operating room techniques used to limit patient exposure to bacteria was a turning point in allowing for the implantation of these devices (King 1959). However, these first devices, made from various alloys available at the time, were susceptible to problems with corrosion (King 1959; Uhthoff, Poitras, and Backman 2006). The development of stainless steel and vitallium (cobalt-chromium-molybdenum alloy) in the early 1900s was a landmark development and starting point for modern metal implant development
From there, the development of metal implantable devices continued to expand into orthopedic, dental, and cardiac areas in the mid20th century (Greatbatch and Holmes 1991; Uhthoff, Poitras, and Backman 2006; Knight, Aujla, and Biswas 2011; Joung 2013; Abraham 2014; Saini et al. 2015). The first generation of hip implants was introduced in the 1950s and 1960s and included metal-on-metal designs with cobalt-chromium alloys (Knight, Aujla, and Biswas 2011).
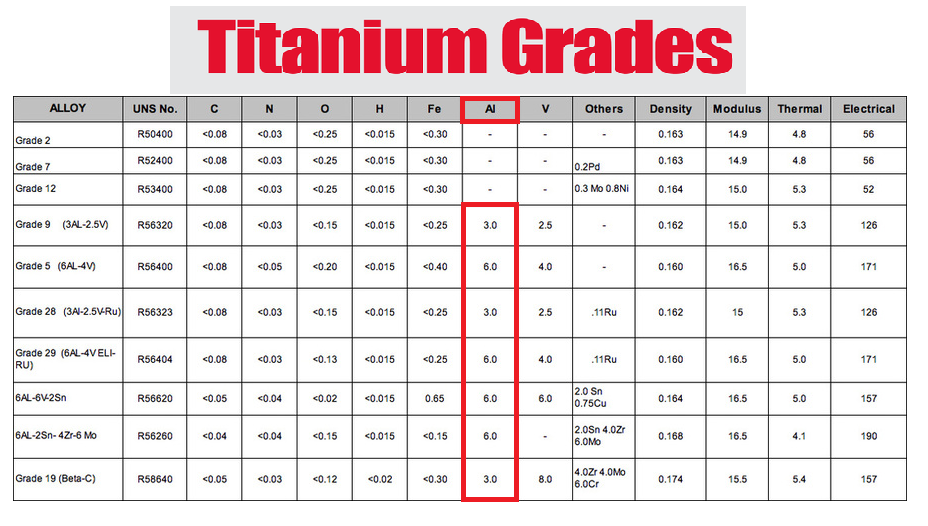
At the same time, the development of miniaturized circuitry allowed for the development of pacemakers, with the first pacemaker being implanted in 1958 (Joung 2013). In subsequent years, advances in technology allowed for metal implants to branch into nearly all medical specialties for the treatment and management of numerous different diseases and conditions. Some developments of note include the first implantable neurostimulator in 1967 (Mullett 1987; Mekhail et al. 2010) and the first balloon-mounted arterial stent in 1985 (Palmaz et al. 1985; Serruys , Kutryk , and Ong 2006). The most common metals used in implants have historically been stainless steel (iron-based alloys), cobalt-based alloys (Co-based), pure titanium, and titanium-based alloys (Ti-6Al-4V).
Various refractory metals (metals which are difficult to fuse or corrode), such as molybdenum (Mo), tungsten (W) and tantalum (Ta), have also been used as alloying elements in implantation materials. Noble metals, such as gold (Au), silver (Ag), platinum (Pt), and iridium (Ir), are common in implants with electronic components (Khan et al. 2014). The use of a particular metal or alloy depends on the application; for example, surgical grade stainless steel is known for its high strength and good ductility, but can be difficult to integrate with bone or soft tissue (Khan et al. 2014). As a result, stainless steel is commonly used in fracture fixation devices and/or temporary implants intended to be removed at a later time.

On the other hand, Co-based alloys (e.g., Co-Cr-Mo, Co-Cr-W-Ni) are highly corrosion resistant, have higher strength and hardness, but have lower ductility and are harder to work and configure by machine (Khan et al. 2014; Matusiewicz 2014). Therefore, Co-based alloys are used more widely in longer-term permanent implants and those that require high wear resistance, such as artificial joints or hip prostheses (Khan et al. 2014). The alloy known as nitinol (Ni-Ti) is being used more frequently in implants due to its shape memory behavior (i.e., ability to return to its original shape after a temperature change) (Elahinia et al. 2012), its superelasticity (i.e., ability to return to its original shape after removal of mechanical stress), and its biocompatibility when properly passivated. Ni-Ti is now used extensively in vascular stents and is being used in implants in various other applications such as orthopedic fixation devices where its unique properties may be advantageous.
Recent developments in materials chemistry, metallurgy, and manufacturing continue to spur innovations in design and diversification in materials utilized in metal implants. For example, additive manufacturing (e.g., 3D printing), is increasingly being utilized to produce custom shapes and geometries using a variety of metals, adding to the potential applications of metal implants.
Because of continued technological development and engineering advances, the number and types of metal implants available on the market has increased in recent years.
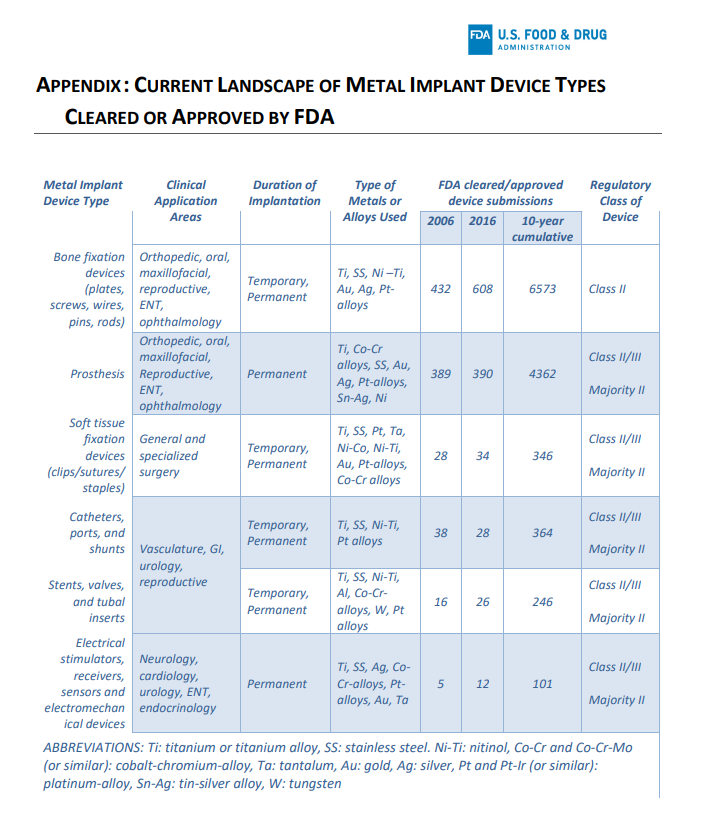
Despite being registered, there are metals and alloys that are safer than others because aluminum has already been proven to have neurotoxic and bioaccumulating effects.
When a healthcare provider prescribes or suggests an implant, ask what type of alloy or if the implant contains aluminum or not.
Consent is important.
Do not buy only by the label, buy by the metal composition.
You will live with this metal for many years, so knowing that is important too.
https://www.fda.gov/media/131150/download
